The most complete paper on petroleum
Minggu, 19 Maret 2023
The most complete paper on petroleum
CHAPTER I
INTRODUCTION
Background
Energy sources that are widely used for cooking, motor vehicles and industry come from petroleum, natural gas and coal. These three types of fuel come from weathering the remains of organisms so they are called fossil fuels. Oil and natural gas come from microorganisms, dead plants and animals.
The remains of these organisms settle to the bottom of the earth and are then covered with mud. The mud gradually turns into rock due to the pressure of the layers above it. Meanwhile, with increasing pressure and temperature, anaerobic bacteria decompose the remains of the microorganisms into oil and gas. Apart from fuel, oil and natural gas are important industrial materials. Materials or products made from oil and natural gas are called petrochemicals. Today tens of thousands of types of petrochemical materials can be classified into plastics, synthetic fibers, synthetic rubber, pesticides, detergents, solvents, fertilizers, and various types of drugs.
Writing purpose
The purpose of writing this paper is:
Can know and deepen the author's knowledge related to petroleum.
Can know the benefits and uses of petroleum for human life.
CHAPTER II
DISCUSSION
Formation of Petroleum
The process of forming petroleum is explained based on two theories, namely:
Inorganic Theory
The inorganic theory was put forward by Berthelok (1866) who stated that petroleum originates from the reaction of calcium carbide, CaC2 (and the reaction between carbonate rocks and alkali metals) and water to produce acetylene which can turn into petroleum at high temperatures and pressures.
CaCO3 + Alkali → CaC2 + HO → HC = CH → Petroleum
Organic Theory
The organic theory was put forward by Engker (1911) which stated that petroleum is formed from the process of weathering and anaerobic decomposition of microorganisms (microorganisms) from marine plants in porous rocks.
Composition of Petroleum
The composition of petroleum is grouped into four groups, namely:
Saturated Hydrocarbons (alkanes)
Known as alkanes or paraffins
The existence of straight chains as the main component (most), while the branched chains are less
The constituent compounds include:
Methane CH4
etana CH3 CH3
propana CH3 CH2 CH3
butana CH3 (CH2)2 CH3
n-heptane CH3 (CH2)5 CH3
isooctane CH3 – C(CH3)2 CH2 CH (CH3)2
Unsaturated Hydrocarbons (alkenes)
Known as alkenes
There are only a few
Compounds:
Pause, CH2 CH2
Propena, CH2 CH CH3
Butene, CH2 CH CH2 CH3
Cyclic Chain Saturated Hydrocarbons (cycloalkanes)
Known as cycloalkanes or naphthenes
Its presence is less than alkanes
Constituents:
Cyclopropane 3. Cyclopentane

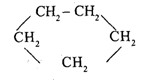
Cyclobutane 4. Cyclohexana
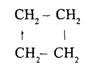

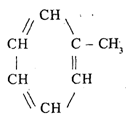
Aromatic hydrocarbons
Known as the aromatic series
Its existence as a small component
Compound composition:
Naphthalene 3. Benzene
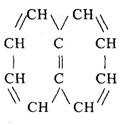

Anthracene 4. Toluene
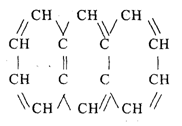
Other Compounds
There are very few of them
Compounds that may exist in petroleum are sulfur, nitrogen, oxygen and metal organo (very small)
Petroleum Processing
Crude oil (Crude oil) obtained from drilling is in the form of a thick black liquid which must be processed first. Oil drilling in Indonesia is located on the north coast of Java (Cepu, Wonokromo, Cirebon), Sumatra (Aceh, Riau), Kalimantan (Tarakan, Balikpapan) and Irian (Papua). Processing of petroleum through two stages, including:
first Processing,
At this stage, "multilevel distillation" separates petroleum fractions based on their boiling points. The component with the higher boiling point will remain a liquid and sink to the bottom. While the lower boiling point will evaporate and rise to the top through cavities called bubble cavities.
second processing,
At this stage it is an advanced process of multilevel distillation results with the following process:
Cracking
Extraction
Crystallization
Cleaning from contamination
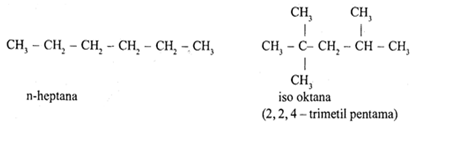
Gas
The composition of gasoline consists of n - heptane and iso octane, namely:
Gasoline Additives
Tetra Ethyl Leat (TEL)
Molecular formula of Pb (C2H5)4
Structural formula
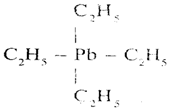
Ethyl Tertier Butil Eter (ETBE)
Molecular formula CH3 OC(CH3)3Tertiary Amyl Methyl Ether (TAME)
Molecular formula CH3 OC(CH3)2 C2H5Methyr Tertiary Butyl Ether (MTBE)
The molecular formula is CH3 OC(CH3)3
Petrochemical
Apart from being a fuel, petroleum is also a chemical industry material that is important and useful in everyday life. Materials or products made from oil and natural gas are called petrochemicals. Petrochemical materials can be classified: plastics, synthetic fibers, synthetic rubber, pesticides, detergents, solvents, fertilizers, various types of drugs and vitamins.
Petrochemical Basic Materials
The petrochemical process generally goes through three stages, namely:
Converting oil and natural gas into petrochemical base materials
Converting petrochemical base materials into intermediate products, and
Converting intermediate products into usable final products.
Almost all petrochemical products come from three types of basic materials, namely:
Olefins (alkenes)
The most important olefins are ethylene (ethylene), propene (propylene), butene (butylene) and butadiene.
CH2=CH2 CH2=CH-CH3
Ethylene propylene
CH3-CH=CH-CH3 CH2=CH-CH=CH2
Butilena butadiena
Aromatics (benzene and its derivatives)
The most important aromatics are benzene (C6H6), totuene (C6H5CH3) and xylene (C6H4 (CH3)2
Synthesis Gas
Synthetic gas is also called syn-gas which is a mixture of carbon monoxide (CO) and hydrogen (H2). Syn-gas is made from the reaction of natural gas or LPG through a process called stean reforming or partial oxidation.
Stean reforming reaction: CH4(g) + H2O → CO(g) + 3H2(g)
Partial oxidation reaction : 2CH4(g) + O2 → 2CO(g) + 4H2(g)
Petrochemicals from Olefins
The following are some of the petrochemicals of ethylene-based olefins:
Polyethylene
Polyethylene is the most widely produced plastic used as plastic bags and plastic wrap/garbage.
PVC
PVC is polyvinylchloride which is a plastic for making pipes (pralon).
Ethanol
Ethanol is an ingredient that we know as alcohol which is used for fuel or as an ingredient in other products.
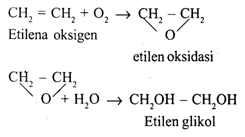
Alcohols are made from ethylene:
CH2 = CH2 + H2O → CH3 – CH2OH
Ethylene glycol or Glycol
Glycol is used as an antifreeze in car radiators in cold climates.
Following are some of the petrochemicals of olefins with propylene as ingredients.
Polypropylene
Polypropylene plastic is stronger than polyethylene. This type of polypropylene plastic is often used for plastic sacks and plastic straps.
glycerol
This substance is used as a cosmetic ingredient (moisturizer), food industry and materials for making explosives (nitroglycerin).
Isopropyl alcohol
This substance is used as the main ingredient for other petrochemical products such as acetone (a solvent, for example to dissolve nail polish).
Petrochemicals whose manufacture uses butadiene as a base material are synthetic rubbers such as SBR (styrene-butadilene-rubber) and nylon -6.6, while those that use isobutylene as a base material are MTBE (methyl tertiary butyl ether).
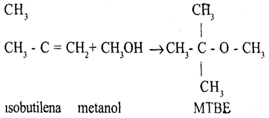
Petrochemicals from Aromatics
The most important aromatic bases are benzene, toluene, and xylene (BTX). The basic ingredients of benzene are generally converted to styrene, cumene and cyclohexane
Styrene is used to make synthetic rubber
Cumene is used to make phenol, followed by phenol to make adhesives
Cyclohexane is used primarily to make nylon
Benzene is used as a base material for making detergents. The base material for toluene and xylene to make explosives (TNT), terephthalic acid (an ingredient in fibres).
Petrochemical and gas-synthetic
Synthetic gas is a mixture of carbon monoxide and hydrogen. Some petrochemical examples of syn-gas are as follows:
Amonia (NH3)
N2(g) + 3H2(g) → 2NH3(g)
Nitrogen gas from the air and hydrogen gas from syn-gas. Ammonia is used to make fertilizer [CO(NH2)2] urea, [(NH4)2SO4]; ZA fertilizer and (NH4NO3); ammonium nitrate.
Urea [CO(NH2)2]
CO2(g) + 2NH3(g) → NH2COH4(S)
NH2CONH4(S) → CO(NH2)2(S) + H2O(g)
Methanol (CH3OH)
CO(g) + 2H3(g) → CH3OH(g)
Most of the methanol is converted to formaldehyde and some is used to make fibers and fuel blends.
Formaldehyde (HCHO)
CH3OH(g) → HCHO(g) + H2(g)
Formaldehyde in water is known as formalin which is used to preserve biological preparations.
CHAPTER III
CLOSING
Conclusion
The process of forming petroleum is derived from the reaction of calcium carbide, CaC2 (from the reaction between carbonate rocks and alkali metals) and water which produces acetylene which can turn into petroleum at high temperatures and pressures.
Apart from fuel, petroleum is also a chemical industry material that is important and useful in everyday life, which is called petrochemical.
BIBLIOGRAPHY
Ika Ratna Sari, S.Pd. 2006. Effective Chemistry Learning Methods: Central Java. CV Media Karya Putra.
Ancient Michael. 2004. Chemistry for SMA: Jakarta. Erlangga PT.
source: ambo
